Vigilance
The safety of patients is a priority. The Corlife is very interested in feedback from treating physicians after implantation. This is the best way we can identify potential for improvement and reduce risks in good time.
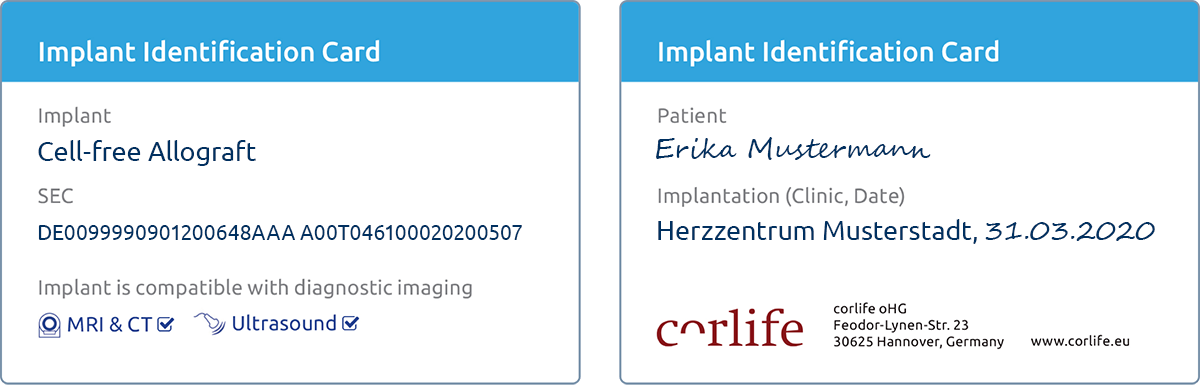
Implant-ID and SEC
Each cell-free allograft is accompanied by an implant identification card. The card shows the serial number of the cell-free allograft (Single European Code, SEC). Use the SEC to learn more about cell-free allograft here . Please present the implant ID card to the patient. The ID card should be kept in a safe place and be available in case of need.

Follow-up report
SALAMANDRA Study

Corlife is funding an independent study on the long-term safety and efficacy of cell-free allografts. This study is coordinated by the Hannover Medical School. Participation is voluntary for both patients and participating physicians.
Please connect with sarikouch.samir@mh-hannover.de, if you want to join SALAMANDRA.
Reporting suspected adverse reactions
Adverse reactions are harmful and unintended reactions to the cell-free allograft. Reporting adverse reactions helps to provide more information on the safety of cell-free allografts:
- Please inform your patients that they should contact their doctor or healthcare professional if they notice any adverse reaction.
- Patients should report any suspected adverse reaction to the competent authority . This also applies to adverse reactions that are not mentioned in the package leaflet. Medical professionals please report here.