Cell-free Human Truncus Pulmonalis, Espoir TP
The "Cell-free Human Truncus Pulmonalis, Espoir TP" is approved by the German competent authority as a medicinal product (PEI.G.11932.01.1) for use in children and adults. The "Cell-free Human Truncus Pulmonalis, Espoir TP" is used for the replacement or reconstruction of the truncus pulmonalis according to the clinical indication.
Clinical experience
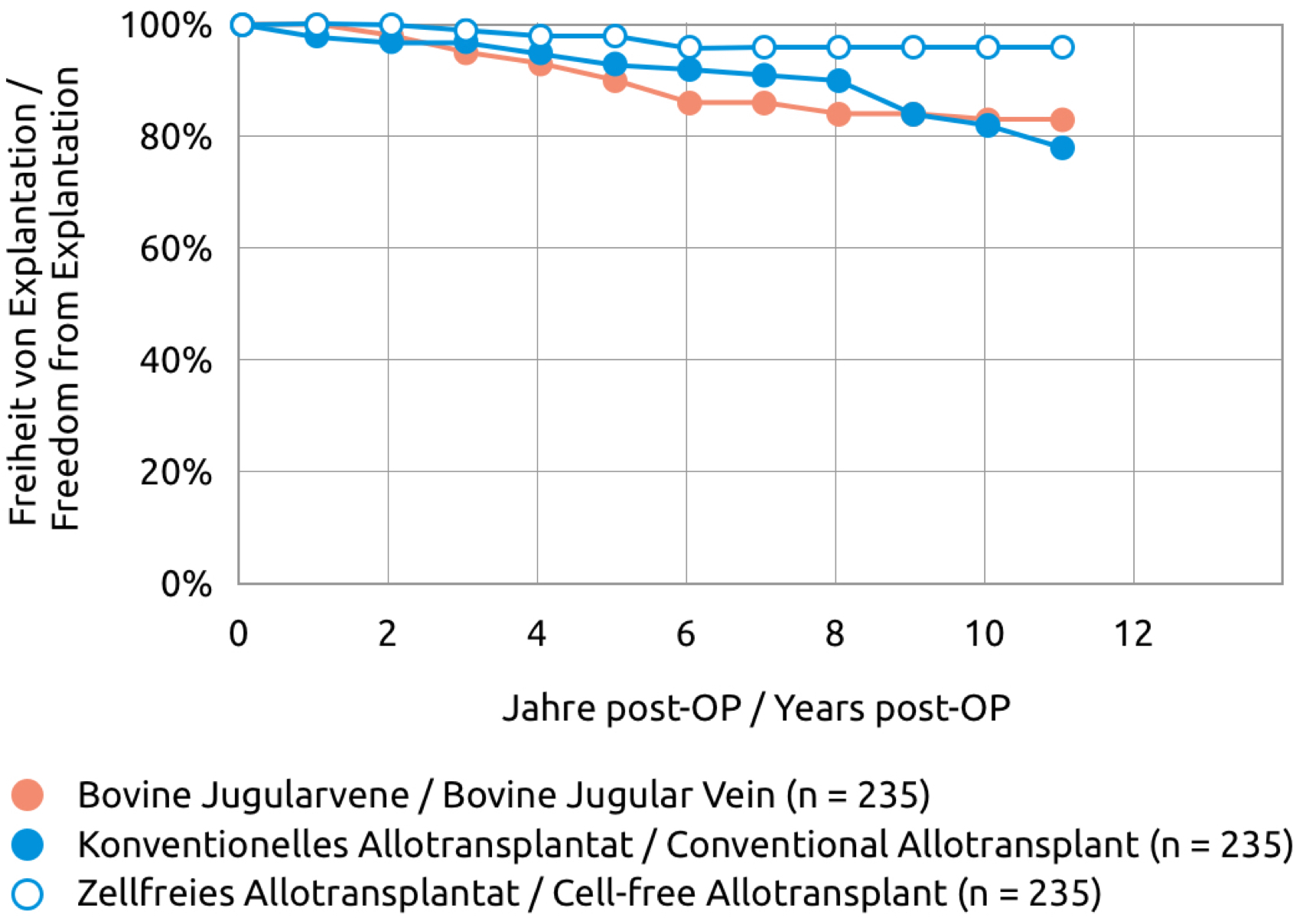
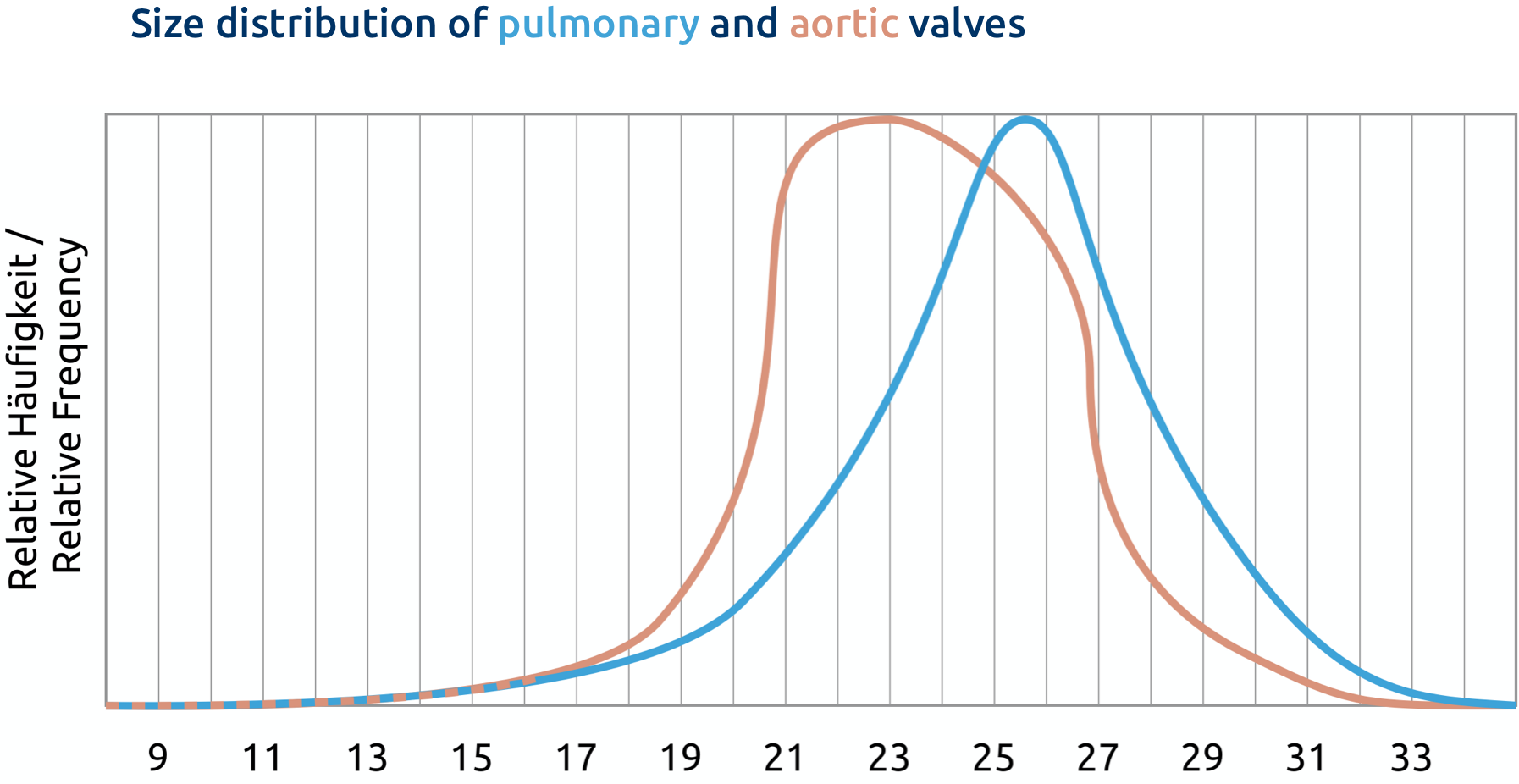
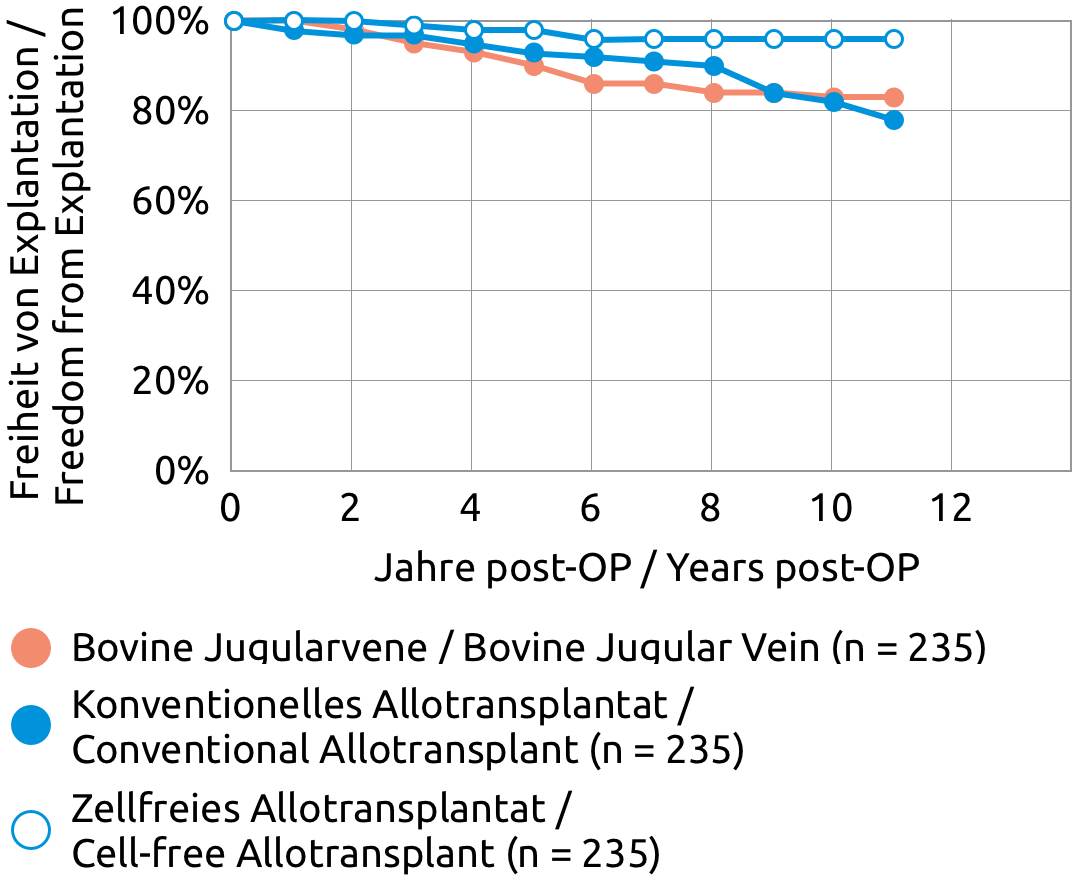
We know from the clinical application of the cell-free pulmonary valve that cell-free pulmonary arteries can be used safely. The frequency distribution for the proximal diameter corresponds approximately to that of the cell-free pulmonary valve.