Cell-free Human Pulmonary Valve, Espoir PV
The "Cell-free Human Pulmonary Valve, Espoir PV" has been approved by the Paul-Ehrlich-Institut as a medicinal product (PEI.G.11634.01.1). It is used to replace or reconstruct a pulmonary valve according to the clinical indication.
Clinical experience
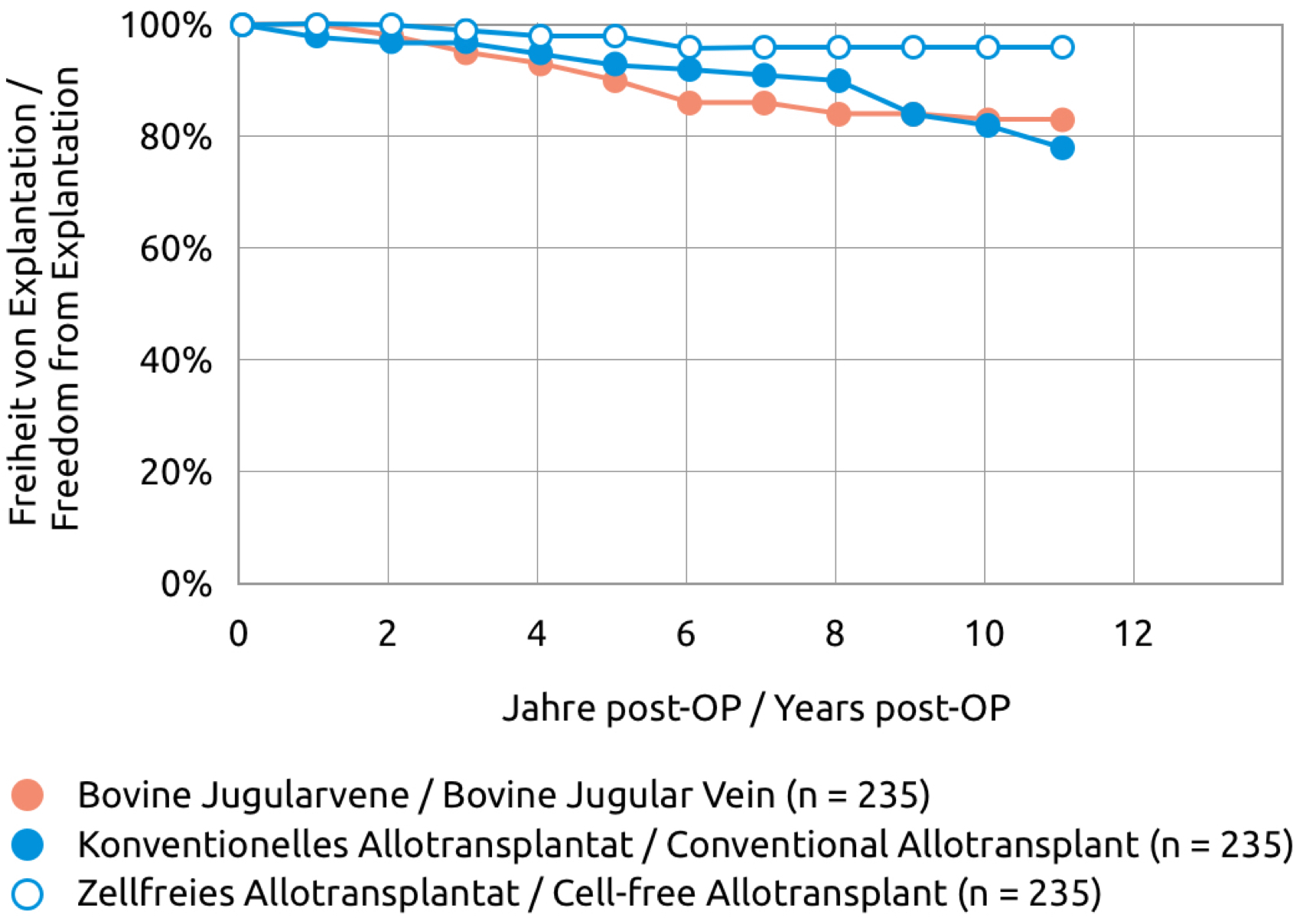
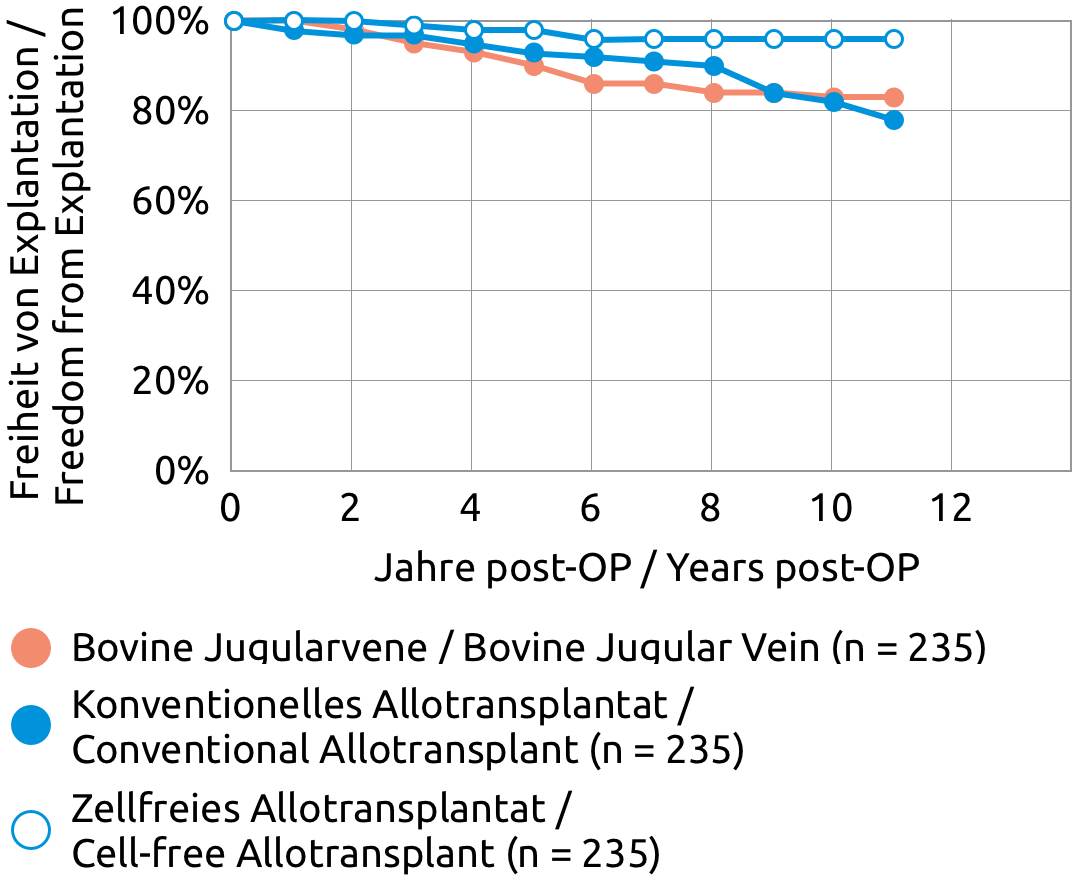
The "Cell-free Human Pulmonary Valve, Espoir PV" can be used safely. Clinically relevant degeneration or calcification has rarely been reported. This also applies to implantations in children and young adults.

Relevant publications
Boethig D, Horke A, Hazekamp M, Meyns B, Rega F, Van Puyvelde J, Hübler M, Schmiady M, Ciubotaru A, Stellin G, Padalino M, Tsang V, Jashari R, Bobylev D, Tudorache I, Cebotari S, Haverich A, Sarikouch S. A European study on decellularized homografts for pulmonary valve replacement: initial results from the prospective ESPOIR Trial and ESPOIR Registry data. Eur J Cardiothorac Surg. 2019 Sep 1; 56(3):503-509. doi: 10.1093/ejcts/ezz054. PMID 30879050
Bobylev D, Sarikouch S, Tudorache I, Cvitkovic T, Söylen B, Boethig D, Theodoridis K, Bertram H, Beerbaum P, Haverich A, Cebotari S, Horke A. Double semilunar valve replacement in complex congenital heart disease using decellularized homografts. Interact Cardiovasc Thorac Surg. 2019 Jan 1;28(1):151-157. doi: 10.1093/icvts/ivy212. PMID 30016427